


All Link may be accessed from QR code.
In addition to basic details of AMED, please take a look at various information such as project introductions, public solicitation like calls for proposals, events, and more.

Please follow us! We post news on public solicitation like calls for proposals, events, and more.

Please subscribe! Recordings from symposium and more are available.

Please subscribe! We deliver information such as public solicitation like calls for proposals and events.

Please follow us! Very clear introductions of medical research and development promoted by AMED!

| 2024.6.27 | We have started accepting registration for participation. |
|---|
You may download poster of this conference by clicking the button below.
Download
Secretariat of "2023 Outcomes Conference"
(Stage Incorporated)

MISHIMA YoshinaoPresident, Japan Agency for Medical Research and Development (AMED) |
Thank you very much for participating in Practical Research Project for Rare/Intractable Diseases Outcomes Conference hosted by Japan Agency for Medical Research and Development (AMED).
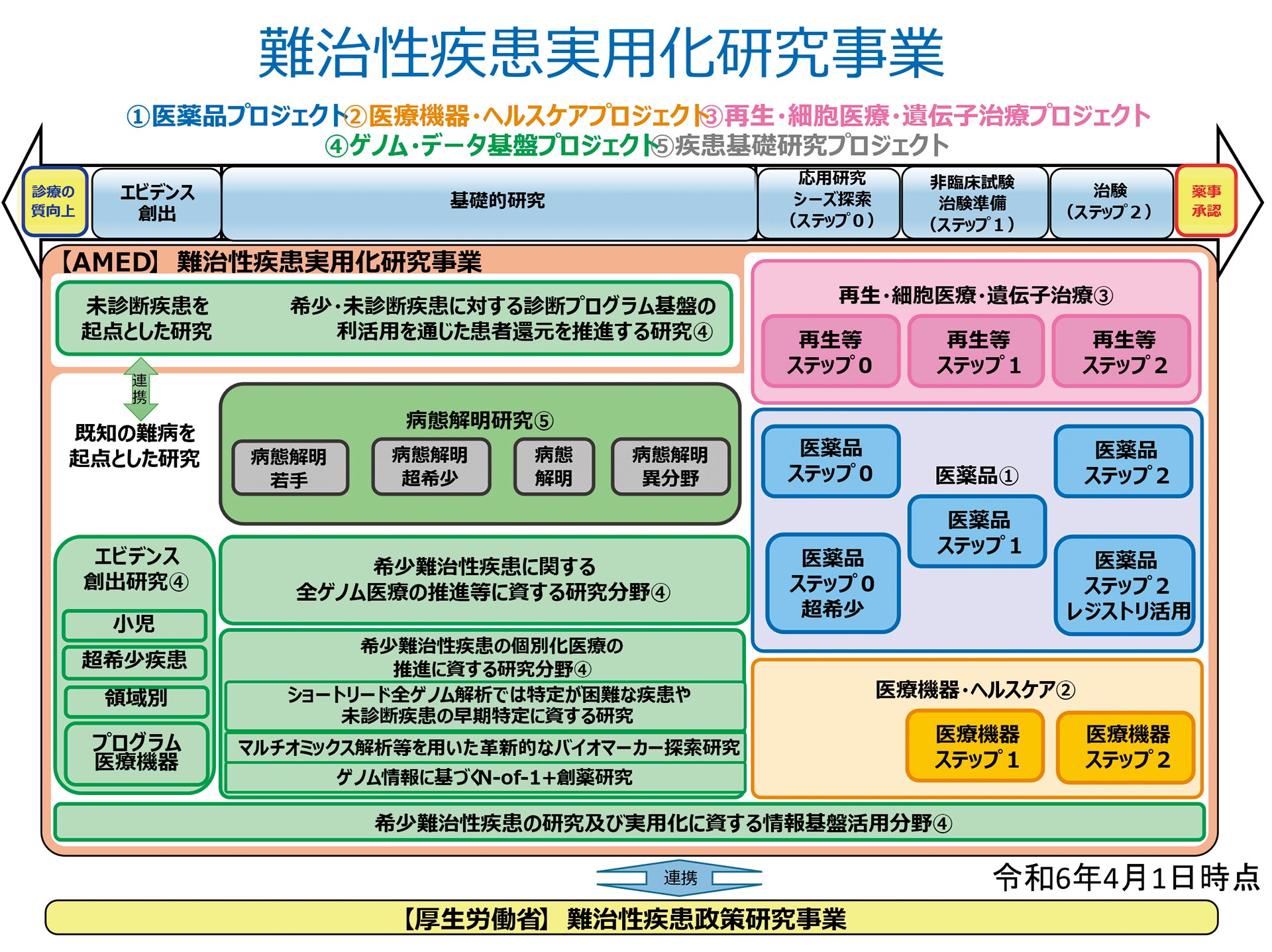
It has been nine years since the establishment of AMED in 2015, and we have been operating more smoothly under the stronger framework of the second medium- to long-term plan, which began in 2020. This second medium- to long-term plan establishes six integrated modality-based projects that are not limited to specific diseases. Among these, the Practical Research Project for Rare/Intractable Diseases supports five integrated projects: Pharmaceuticals, Medical Devices/Healthcare, Regenerative/Cellular Medicine and Gene Therapy, Genome and Data Infrastructure, and Basic Research on Diseases. This support extends seamlessly from basic research to practical application.
The purpose of this outcomes conference is to widely report the research outcomes created in the 2023 fiscal year under the Practical Research Project for Rare/Intractable Diseases to the general public, including patients and their families, and to share information among researchers to further promote research. This year, as in the previous year, the event will be held online. All research outcomes supported by the Practical Research Project for Rare/Intractable Diseases can be viewed on demand at any time during the approximately six-month event period. Additionally, a bulletin board will be set up for each presentation to facilitate Q&A sessions, allowing for interactive information sharing between the presenting researchers and the participants. We also have the "Messages to Patients" section, where researchers convey their thoughts on how they hope to deliver their achievements to patients, their families, and society as a whole. Please take a moment to read their heartfelt messages. Furthermore, on the first day of the event, July 3, we will host a live event inviting people closely related to rare diseases. Various themes, including research and development projects targeting "ultra-rare diseases," which is a first-time attempt for our project, Patient and Public Involvement (PPI) activities, and projects that have made progress towards practical application of the research outcomes, will be introduced.
AMED plays a central role in research and development in the medical field and aims to "quickly bring research outcomes in the medical field to practical use and deliver them to patients and their families." We are acutely aware of the significant role AMED plays in advancing practical applications for rare and intractable diseases.
We would like to thank researchers, patients and their families, companies, and many others for participating in this outcomes conference and for your continued support of the AMED Practical Research Project for Rare/Intractable Diseases.
June 2024

KUSUNOKI SusumuProfessor Emeritus, Kindai University |
I am KUSUNOKI Susumu, the Program Supervisor for the Practical Research Project for Rare/Intractable Diseases (hereafter referred to as "the Project").
In Japan, measures are being taken to address rare and intractable diseases that meet the four criteria of "rarity," "unknown cause," "lack of effective treatment methods," and "long-term disruption to daily life." Following the enactment of the Act on Medical Care for Patients with Intractable Diseases (Act No. 50 of 2014) in 2015, 110 diseases were designated as intractable diseases eligible for medical expense subsidies. By 2021, this number had expanded to 338 diseases. To overcome these intractable diseases, it is necessary to promote basic technological development research for treatment methods, research to establish a research foundation, and pharmaceutical development research.
The Project aims to target rare and intractable diseases, promote the elucidation of their causes and mechanisms, and develop groundbreaking diagnostic, treatment, and prevention methods to overcome them. In collaboration with the Ministry of Health, Labour and Welfare and the research groups of the Policy Research Project on Intractable Diseases, AMED's role is to seamlessly bridge the gap to clinical practice. To achieve this, we promote research and development across five of AMED's six integrated projects: Pharmaceuticals, Medical Devices/Healthcare, Regenerative/Cellular Medicine and Gene Therapy, Genome and Data Infrastructure, and Basic Research on Diseases.
In the 2023 fiscal year, three drug approvals were achieved based on the Project's research and development, demonstrating tangible progress. This Outcomes Conference serves not only as a platform to present the research activities and achievements of the 2023 fiscal year but also as an opportunity for researchers and companies to exchange information, contributing to further research development. We also hope that patients and their families will gain a better understanding of the Project and its various research and development initiatives.
The Project will continue to vigorously promote consistent medical research and development from basic research to practical application, striving to deliver as many research outcomes as possible to patients and their families at the earliest opportunity. We kindly ask for your continued understanding and support of the Project.

ASAI FumitoshiVisiting Professor, Nippon Veterinary and Life Science University |
During my student years, I struggled with the research on skeletal muscle and motor neurons assigned to me by my academic advisor. I felt disinterested in basic research that relied solely on reagents, so I consulted my professor. He advised me, "You've come this far, so try to stick with it and complete your thesis. Perhaps a research theme related to diseases like muscular dystrophy would have suited you better. Why not consider doing drug discovery research at a pharmaceutical company?"
Following my mentor's advice, I joined a pharmaceutical company for drug discovery research, which turned out to be unexpectedly fascinating. I ended up working there for 27 years before transitioning to a university. It was not a smooth journey, but I was fortunate to experience the success of developing several drugs firsthand. The two lessons I learned in the field of drug discovery were: (1) Bringing an idea from a single researcher to fruition as a drug requires the cooperation of many people, including companies, clinicians, patients, and government officials. (2) The success rate of drug discovery is extremely low, so rather than focusing on the causes of failure, it is important to boldly challenge oneself to increase the success rate.
In our country, due to a social climate that hinders the growth of pharmaceutical ventures, the practical research of drugs that major pharmaceutical companies are not interested in, especially for rare disease treatments, is primarily led by university researchers. Whether we view this as a negative—"we have to lead because pharmaceutical companies won't"—or as a positive—"this is a chance for academic researchers to lead drug discovery research (and even win Nobel Prizes)"—can significantly impact the outcome. Personally, through my involvement as a Program Officer (PO) in AMED's intractable disease project, I have felt a great sense of accomplishment each time I meet ambitious and challenge-driven academic researchers. Moreover, every time I am asked to comment on issues related to muscular dystrophy, I feel grateful for the opportunity to give back to the field of skeletal muscle and motor neuron research, which I distanced myself from after obtaining my degree.

IGARASHI TakashiPresident, National Center for Child Health and Development |
Approximately 60% of intractable diseases is considered to be due to genetic abnormalities. According to recent surveys, humans have over 26,000 genes, more than 9,600 hereditary diseases, and about 6,300 identified causative genes (OMIM, April 2022), with these numbers increasing every year. For these intractable diseases with a wide range of causative genes, AMED’s Practical Research Project for Rare/Intractable Diseases employs various approaches to develop treatments, yielding remarkable results and providing great hope for patients suffering from these diseases.
Among the various treatment developments for intractable diseases, regenerative medicine and gene therapy are currently highly anticipated. In the UK and the US, a significant portion of research and development investment in regenerative and gene therapy is directed towards gene therapy. This trend reflects the belief in the effectiveness of gene therapy for treating genetic disorders and cancer. The results of these investments are already beginning to show, with various gene therapy products being put to practical use, primarily in the UK and the US, and now being introduced in Japan. However, in Japan, there are still relatively few researchers specializing in basic and clinical research related to gene therapy. Challenges remain in improving viral vectors, developing new introduction technologies, mass-producing vectors, genome editing techniques, and ensuring safety. The support system to address these challenges is also insufficient.
Through the Project of AMED, we hope to see significant advancements in research on intractable diseases as a whole. Additionally, we wish for further collaboration between AMED and related government agencies to promote not only regenerative medicine but also basic and clinical research in gene therapy.

IKEDA SadakatsuProfessor, Department of Cancer Genomics, Tokyo Medical and Dental University Hospital |
Recent advances in medical science have been remarkable, and even in the field of intractable diseases, groundbreaking gene therapies have been put into practical use, and the latest research and development using cell therapy and AI are being conducted. At AMED, we provide comprehensive support from basic pathological elucidation to the discovery of seeds and the development of pharmaceuticals and medical devices, all aimed at overcoming intractable diseases. We are committed to promoting cutting-edge research and development and strive together with everyone to ensure that as many patients with intractable diseases as possible can benefit from these advancements.

INAGAKI OsamuFormer Secretary of the Steering Committee of Drug Evaluation Committee, Japan Pharmaceutical Manufacturers Association |
To promote medical innovation, the second phase of AMED is organized by modality with a focus on technical perspectives. Within this framework, the "Practical Research Project for Rare/Intractable Diseases" is positioned from a disease perspective and aims to provide new medical treatment for patients with intractable diseases who previously had no appropriate treatments or few treatment options, using a cross-disciplinary approach. This project will bridge research to practice in order to translate new treatment ideas, generated from disease research results, into new treatments through regulatory approval. As a Program Officer, I am pleased to support the promotion of this project so that the innovative treatment ideas of many esteemed researchers can be more quickly translated into society and delivered to patients.

SHIMADA TakashiProfessor Emeritus, Nippon Medical School |
Gene therapy had been stagnant for a while due to issues with side effects, but recently its efficacy in treating many intractable diseases has become evident. In Europe and the United States, it is being developed as the next-generation medical technology, surpassing regenerative cell therapy, and several gene therapy products are already being sold by major pharmaceutical companies at extremely high prices. Unfortunately, gene therapy research in Japan has lagged behind for various reasons. Until now, both the number of gene therapy researchers and research funding have been limited, and there have been few companies involved. Additionally, because clinical viral vectors cannot be produced in Japan, gene therapy for diseases with potential treatment benefits could not be conducted. To overcome this situation and revitalize gene therapy research in Japan, AMED is starting new initiatives in collaboration with academia and industry.
To catch up with and surpass gene therapy in the West, continuous support from basic research to pharmaceutical development is necessary. In the Basic Technology Development Project, efforts are being made to develop manufacturing technologies for viral vectors, establish manufacturing facilities, and set up large animal testing facilities. The Practical Research Project for Rare/Intractable Diseases and the Practical Research for Innovative Cancer Control support gene therapy research targeting specific diseases. Moving forward, it is crucial to increase the number of accepted projects and encourage the participation of young researchers. It is also important to gather gene therapy researchers dispersed across various projects to share information for practical application. Furthermore, collaborating with the regenerative medicine research field, where Japan is leading, to develop unique Japanese gene therapies is essential. Additionally, establishing a supply system for clinical-grade vectors, which is indispensable for clinical development, needs to be urgently arranged through industry-government-academia collaboration. Through these support activities and researchers' efforts, I hope that gene therapy research will progress, enabling the implementation of gene therapy for many diseases in Japan as well.

NARUKAWA MamoruProfessor, Department of Clinical Medicine (Pharmaceutical medicine), Kitasato University Graduate School of Pharmaceutical Sciences |
My name is Mamoru Narukawa, and I am honored to serve as a Program Officer for this project.
In the quest to develop new treatments for intractable diseases, clinical trial data is indispensable for determining whether these treatments should be introduced into medical practice as pharmaceuticals or medical devices. The success of clinical trials is built on the tireless efforts of researchers and industry professionals, along with the invaluable cooperation of many patients. Furthermore, various regulations are in place to ensure that these trials are conducted with the utmost integrity.
Many intractable diseases still lack satisfactory treatment methods. These diseases typically have a small patient population, making it challenging to demonstrate the evidence of effectiveness and safety for new treatments. I specialize in the design and methodology of clinical trials, the analysis and evaluation of the resulting data, and regulations related to pharmaceuticals and clinical trials. Leveraging this knowledge and experience, I aim to support research in the medical field targeting intractable diseases so that practical research can progress smoothly and efficiently, and the resulting research outcomes can be made available to many people as quickly as possible.

NISHIZAWA MasatoyoPresident, Niigata University of Health and Welfare |
The concept of "intractable disease" varies depending on its definition, but it generally refers to diseases with unknown causes and no established treatments, and now includes the criterion of rarity. While advancements in medicine have led to the development of symptomatic treatments for some diseases, removing them from the category of "intractable diseases," many diseases remain progressive and still cannot be controlled.
Research on intractable diseases cannot progress without adequate funding, an increase in researchers, and vibrant activity. While natural science research often begins with the researchers' spontaneous interest and curiosity, the pathology research supported by AMED is driven by a clear goal: to contribute to the development of treatments for patients with intractable diseases. I urge you to always keep this objective in mind as you advance your research. At this year's results presentation, I am very much looking forward to seeing new achievements from your research that will contribute to overcoming these challenging diseases.

WATANABE HiroshiExecutive Director and Vice President, Hamamatsu University School of Medicine |
I have been involved as a Program Officer in the Practical Research Project for Rare/Intractable Diseases since its inception, from the perspective of clinical pharmacology and cardiology. Regarding the drug development for intractable diseases, over twenty years ago, I attempted drug repositioning of a PDE5 inhibitor, originally approved for erectile dysfunction, for pulmonary arterial hypertension. Significant effects on pulmonary hemodynamics and symptoms were observed, and this pioneering application of PDE5 inhibitors for pulmonary arterial hypertension was presented at the American College of Cardiology. However, at that time, Japan lacked a system for physician-led trials, and despite requesting multiple times, pharmaceutical companies did not initiate trials for expanding indications. Looking back, concerns about limited market potential and being a foreign-based company may have hindered conducting trials solely in Japan. Additionally, the evidence we provided was based on case reports rather than randomized controlled trials (RCTs), possibly not reaching the level of evidence required to move pharmaceutical companies. Fortunately, thanks to the interest sparked by presentations at academic conferences, multiple researchers across institutions conducted clinical trials using the same protocol, leading to approval based on domestic data through a public knowledge application for the aforementioned PDE5 inhibitor, albeit delayed. Today, it stands as a first-line treatment for pulmonary arterial hypertension. While robust evidence through RCTs is crucial, completing RCTs for rare diseases often proves challenging. Even at the level of case reports, if a drug objectively improves symptoms or test data for progressive intractable/rare diseases, such observations can constitute excellent evidence. Fortunately, physician-led trials are now feasible. I hope that utilizing rigorous and objective assessment criteria, and employing methodologies such as N-of-1 trials, will enable Japan to generate excellent evidence even with small sample sizes.

WADA KazukoDeputy Director, Osaka Women’s and Children’s Hospital |
Thank you for attending this year's outcomes conference. We deeply appreciate the ongoing efforts of all researchers, healthcare professionals, welfare specialists, government officials, and industry stakeholders involved in intractable disease research.
In this project, a diverse range of studies on many intractable diseases have been adopted, leading to active research endeavors. The collaboration among researchers from various fields fosters swift sharing of the latest research findings and insights, stimulating each other to accelerate advancements in intractable disease treatments.
Moreover, Public and Patient Involvement (PPI) is recognized as a key aspect of medical research. Particularly in intractable disease research, the involvement of patients and their families is indispensable. Incorporating direct input from patients and citizens is highly effective in enhancing the direction and quality of research. Understanding patients' experiences and needs, and gaining their cooperation, makes research more practical and ultimately contributes to the development of effective drug and treatments. In this regard, we hope that this outcomes conference serves not only as a platform for exchange among researchers but also as a forum for sharing information and exchanging views among researchers, patients, their families, and the community.